Research & Development at Nielsen Biosciences
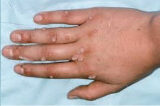
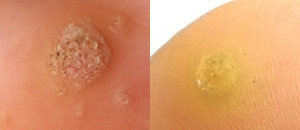
Nielsen BioSciences’ R&D efforts are focused on exploring the potential of its purified candida antigen CANDIN for the treatment of human papilloma virus (HPV) – driven indications. Specifically, Nielsen is studying CANDIN for the treatment of verruca vulgaris or common warts. CANDIN is currently not approved for the treatment of common warts, but is approved as a skin test antigen for the assessment of cellular hypersensitivity to Candida albicans.
Nielsen BioSciences initiated a rigorous clinical program to investigate the potential of CANDIN in the treatment of common warts. Results of the Phase 2 research were highly encouraging and provided the foundation for the current Phase III program. The results of the Phase II study were peer-reviewed and published in Dermatology & Therapy. See Randomized Phase IIa Trial of Purified Candida Antigen for Common Warts: Evaluating the Safety and Efficacy Across Multiple Dosing Regimens | Dermatology and Therapy
The consecutive Phase III clinical trial is fully recruited. The primary endpoint is assessing CANDIN’s potential to provide complete resolution of treated warts without recurrence. Several secondary endpoints are also being studied. Information on our ongoing clinical trials can be obtained at https://clinicaltrials.gov/search?term=NCT05889845. This Phase III multi-center randomized, double-blind, placebo-controlled study is currently the only Phase III clinical study program to investigate treatment of warts. The study is fully enrolled with patients in the US and Japan and projected to report results in Q4 2025.
In addition, based on the encouraging results of our clinical program and a significant body of peer reviewed publications, we plan to continue evaluating the Candin antigen active ingredient as a potential treatment for a variety of other viral dermatological conditions, including Molluscum Contagiosum, Plantar Warts, Periungual Warts, Flat Warts, Facial Warts, and Genital Warts
Pipeline