Research & Development at Nielsen Biosciences
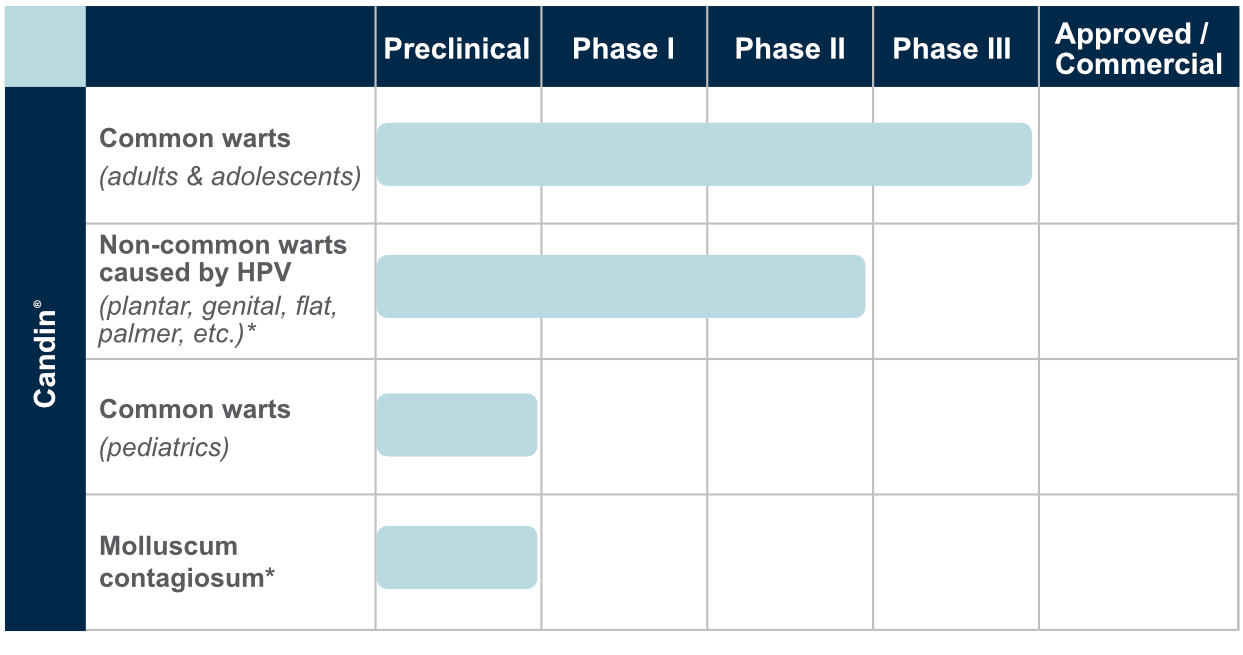
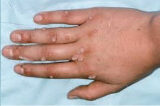
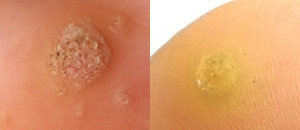
The active ingredient in our CANDIN product, currently approved as a skin test antigen for the assessment of cellular hypersensitivity to Candida albicans, is currently under investigation for the treatment of Verruca vulgaris or common warts. The antigen in CANDIN is not currently approved for the treatment of common warts but we are exploring whether this biologic product may provide an opportunity to leverage the body’s immune response to more effectively address that condition. Nielsen BioSciences has initiated a Phase 3 clinical trial for evaluation in the treatment of human papilloma virus (HPV) conditions including Verruca vulgaris or common warts.
This program is investigational: CANDIN is not currently approved for the treatment of common warts.
Information on our ongoing clinical trials can be obtained at https://clinicaltrials.gov/search?term=NCT05889845
Results in Phase 2 research involving common and non-common warts among adults were highly encouraging, and we are now advancing clinical research into use of the antigen in common and non-common warts. Though not treated, Plantar, Periungual, Flat, Facial, and Genital warts were evaluated in the Phase 2 subjects.
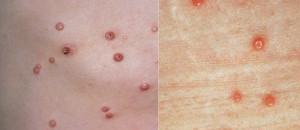
In addition, based on the Phase 2 results and a significant body of peer reviewed publications, we plan to continue evaluating the Candin antigen active ingredient as a potential treatment for a variety of other viral dermatological conditions, including Molluscum Contagiosum, Plantar Warts, Periungual Warts, Flat Warts, Facial Warts, and Genital Warts
Drug Development Pipeline